Hydrogen production methods
Hydrogen can be a versatile fuel and it can be produced through thermal processes, electrolytic processes, solar-driven processes and biological processes.
Thermal Processes
– Natural gas reforming
– Coal gasification
– Biomass gasification
– Reforming of liquid fuels
The most prevalent techniques include natural gas reforming, which involves high-temperature steam reforming where steam reacts with hydrocarbon fuels to produce hydrogen. This method accounts for approximately 95% of current hydrogen production and can utilize a range of hydrocarbon sources, such as natural gas, diesel, gasified coal, or gasified biomass.
Example of chemical reaction of the reforming process:
Steam-methane reforming reaction
CH4 + H2O (+ heat) → CO + 3H2
Water-gas shift reaction
CO + H2O → CO2 + H2 (+ small amount of heat)
Although natural gas reforming is a widely used method for hydrogen production, it does have drawbacks, including its energy intensity and the production of carbon monoxide (CO) and carbon dioxide (CO2) as byproducts. It is by no means a carbon neutral solution even if the combustion of hydrogen itself is considered “clean”.
While recent efforts have largely focused on scaling up hydrogen production, hydrogen already is an important industrial gas, with dedicated annual production of 70 million tons. It’s also a significant user of fossil fuels globally, consuming roughly 6 percent of natural gas supply and 2 percent of coal supply. Hydrogen production from natural gas reforming and coal gasification generate substantial emissions. Currently, the dedicated production of hydrogen emits 830 million tons of CO2, which represents more than 2 percent of global fossil CO2 emissions.
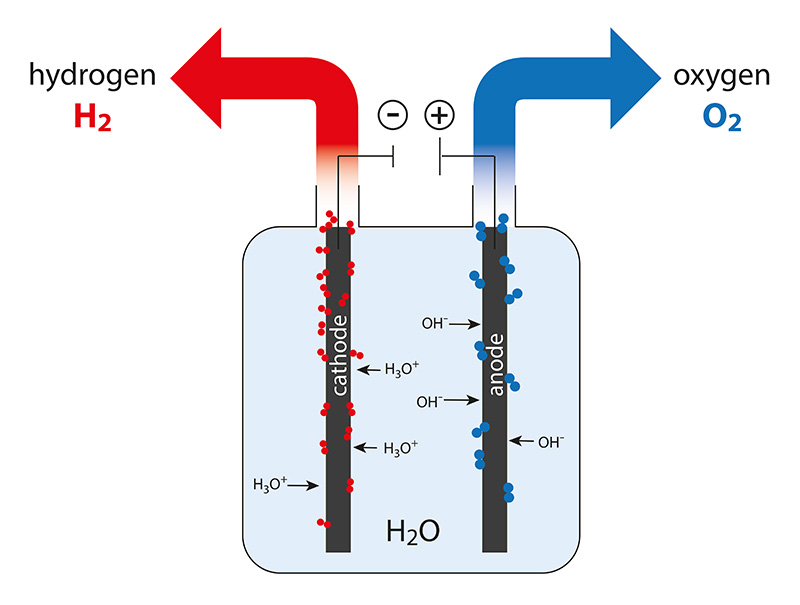
Electrolytic Processes
Electrolysis is the process of splitting water into oxygen and hydrogen using an electricity. This reaction takes place in a unit called an electrolyzer.
This process operates in reverse to a fuel cell, creating hydrogen from water molecules instead of utilizing the energy of hydrogen molecules.
It is important to note. Today’s grid electricity is not the ideal source of electricity for electrolysis because most of the electricity is generated using technologies that result in greenhouse gas emissions and are energy intensive. In addition, it requires a lot of water as well. While many proposals suggest the use of ocean water, which is plenty full, we have similar issues as we do with desalination processes. It may also lead to more industrialization of coastal areas as well as other bodies of water.
Solar-Driven Processes
Solar-driven processes employ light as the catalyst for hydrogen production. Several solar-driven techniques exist, including photobiological, photoelectrochemical, and solar thermochemical processes. Photobiological processes utilize the natural photosynthetic activity of bacteria and green algae to produce hydrogen. Photoelectrochemical processes utilize specialized semiconductors to separate water into hydrogen and oxygen. Solar thermochemical hydrogen production employs concentrated solar power to drive water splitting reactions, often in conjunction with other substances like metal oxides.
These processes are still being researched and have not been able to be scaled up.
Biological Processes
Biological processes involve the use of microorganisms such as bacteria and microalgae to produce hydrogen through biological reactions. In microbial biomass conversion, microbes break down organic matter like biomass or wastewater to generate hydrogen. In photobiological processes, microbes utilize sunlight as the energy source for hydrogen production.
These processes are still being researched and have not been able to be scaled up.